Antibiotic resistance has become a critical issue in healthcare, especially concerning common pathogenic bacteria like Klebsiella pneumoniae. This opportunistic pathogen poses significant challenges due to its ability to develop multidrug-resistant phenotypes, particularly in patients with diabetic foot ulcers (DFU). Recent research sheds light on the genetic characterization of this resistance, offering valuable insights into combating this growing threat.
The Rise of Antibiotic Resistance in Klebsiella pneumoniae
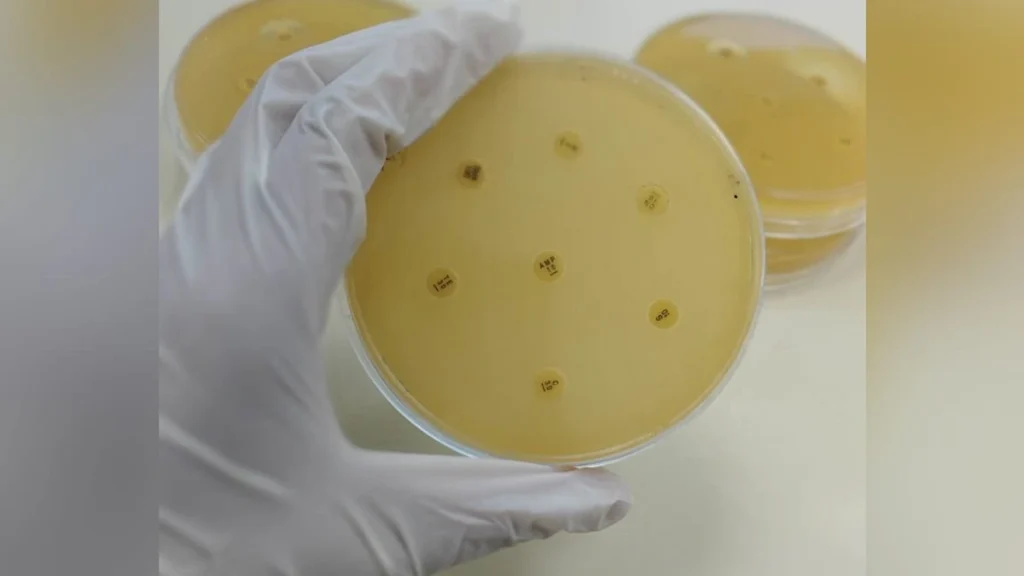
The study conducted on the western Indian population highlights the alarming prevalence of antibiotic resistance in Klebsiella pneumoniae isolates from DFU patients. Of the 161 isolates analyzed, 50.93% were positive for Extended-Spectrum Beta-Lactamase (ESBL) production, and 45.96% for Metallo-Beta-Lactamase (MBL) production. These enzymes are responsible for breaking down a wide range of antibiotics, rendering them ineffective.
Genetic Basis of Antibiotic Resistance
Antibiotic resistance is often linked to the genetic makeup of bacteria. In this study, researchers identified several key genes responsible for ESBL and MBL production. The TEMESBL gene was the most prevalent, followed by CTX-MESBL, NDM-1bla, and SHVESBL. The least common was the VIMbla gene. Interestingly, there was no direct correlation between the presence of these genes and the observed antibiotic resistance, indicating that multiple factors contribute to resistance mechanisms.
Implications for Treatment and Public Health
The findings underscore the need for continuous monitoring and management of antibiotic resistance in clinical settings. As most isolates were resistant to commonly used antibiotics like Ampicillin and Amoxicillin, healthcare providers must consider alternative treatment options. Imipenem and Amikacin showed partial effectiveness, offering potential avenues for therapeutic intervention.

Conclusion
The emergence of antibiotic resistance in Klebsiella pneumoniae from diabetic foot ulcers represents a significant threat to public health. This study highlights the complex genetic mechanisms underlying resistance and the urgent need for innovative solutions to combat this issue. Continued research and global collaboration are essential to developing effective strategies to manage and mitigate the impact of antibiotic-resistant infections.
By staying informed and proactive, we can work towards a future where antibiotic resistance is effectively managed, ensuring better health outcomes for all.
To read full article follow this link https://journals.lww.com/jpbs/fulltext/2024/16003/genetic_characterization_of_extended_spectrum.200.aspx